Annexin V-FITC Apoptosis Detection Kit
Features
- Annexin V-FITC Apoptosis Detection Kit contains ready-to-use solutions, Annexin V-FITC conjugate, propidium iodide (PI).
- The kit can identify apoptotic and necrotic cells
- Detect by flow cytometry or fluorescence microscopy
- No need to fix cells
Product Information
Mechanism
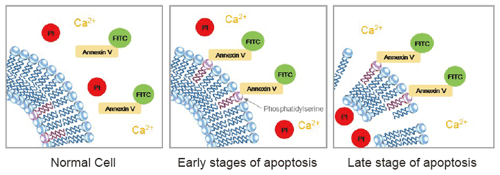
In normal cells, phosphatidylserines (PS, membrane phospholipids) are held on the inner layer of the cell membrane, so Annexin V does not attach to the cells. During early apoptosis, the PS are exposed on the outer layer, where they attach to the FITC-labeled Annexin V and stain the cell surface green. During late apoptosis, propidium iodide (PI) enters the cell and stains the contents red.
Application 1
Fluorescent imaging of apoptosis induced cells
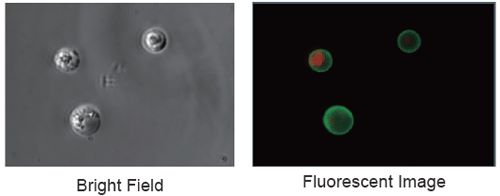
Jurkat cells were apoptosis induced with staurosporine
with its concentration of 1 µg/ml at 37 °C for 3.5 hours
and then observed under a fluorescent microscope.
FITC-labeled Annexin V (Green)
PI (Red)
Application 2
Flow cytometric analysis of apoptosis induced cells
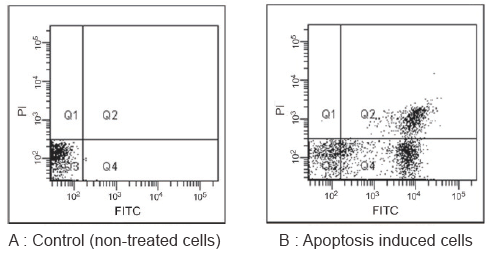
Jurkat cells were apoptosis induced with staurosporine (1 µg/ml) at 37 °C for 3.5 hours and then analyzed with a flow cytometer.
Components
| Reagents | for 100 assays | |
|---|---|---|
| Volume | Quantity | |
| Annexin V-FITC Conjugate | 250 µl | 2 |
| PI Solution | 250 µl | 2 |
| Annexin V Binding Buffer (10x) | 10 ml | 2 |
Applications
Apoptosis Detection Kit Performance Comparison
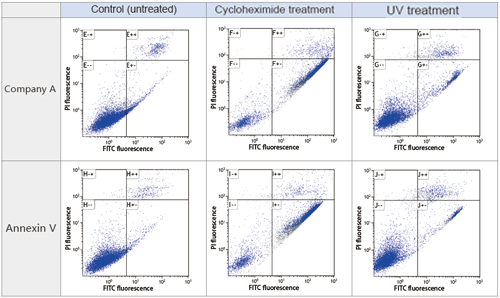
Cell type: Mouse B16 melanoma cells
Number of cells: 10,000
Instrument: Gallios (Beckman Coulter Inc.)
Apoptosis was induced by cycloheximide (1 µg/ml) or UV irradiation at 37°C for 3.5 hours. Cells were analyzed by flow cytometry. Our product showed about the same performance as the product from company A. The control cells did not display any signs of apoptosis. Treatment with cycloheximide resulted in many cells in early apoptosis. UV irradiation also induced apoptosis in the tested cells.
--: FITC-labeled Annexin V and PI both had low fluorescence values. Live cells.
+-: FITC-labeled Annexin V had high fluorescence value, PI was low. Early apoptosis.
++: FITC-labeled Annexin V and PI both had high fluorescence values. Late apoptosis.
Data Courtesy of Assistant professor Yuuki Takahashi , Graduate School and Faculty of Pharmaceutical Sciences, Kyoto University
Apoptosis Detection Kit Performance Evaluation
| Control (untreated) | Starvation (48 hours) |
|---|---|
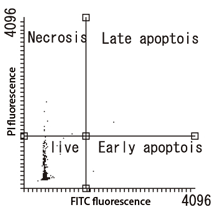 |
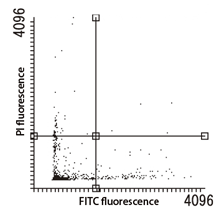 |
Cell type: U-87 MG cells
Instrument: Tali® Image-Based Cytometer (Thermo Fisher Scientific Inc.)
Apoptotsis was induced by removing glucose and starving the cells for 48 hours. Upon analysis using an imaging cytometer, some cells were observed in early apoptosis.
Data Courtesy of Assistant Professor Hitoshi Gotoh Department of Biology / Developmental Neurobiology, Liberal Arts and Sciences, Department of Biology, Kyoto Prefectural University of Medicine
Protocols
Preparation
Annexin V Binding Solution
Dilute Annexin V Binding Buffer (10x) 10-fold with distilled water.
Protocols
General Protocol for Suspension Cells
- Centrifuge the cell suspension at 1,000 rpm for 3 minutes and remove supernatant.
- Add PBS to wash cells and centrifuge at 1,000 rpm for 3 minutes, remove supernatant. (Do this step twice.)
- Add 10-fold diluted Annexin V Binding Solution to make final cell concentration of 1 x 106 cells/ml.
- Transfer 100 µl of cell suspension prepared in step 3 to a new tube.
- Add 5 µl of Annexin V - FITC Conjugate, then 5 µl of PI Solution to the cell suspension.
- Incubate 15 minutes at room temperature with protect from light.
- Add 400 µl of 10-fold diluted Annexin V Binding Solution.
- Apply the solution prepared in step 7 to flow cytometric assay or microscopic assay.
General Protocol for Adherent Cells
- Discard supernatant on the petri dish or plate.
- Add PBS for wash cells and discard supernatant. (Do this step twice.)
- Detach the cells with Trypsin-EDTA.
- Add appropriate volume of culture medium or PBS and transfer the cell suspension to a tube.
- Centrifuge at 1,000 rpm for 3 minutes. Remove supernatant.
- Add PBS to wash cells and centrifuge at 1,000 rpm for 3 minutes, remove supernatant. (Do this step twice.)
- Add 10-fold diluted Annexin V Binding Solution to make final cell concentration of 1 x 106 cells/ml.
- Transfer 100 µl of cell suspension prepared at step 7 to a new tube.
- Add 5 µl of Annexin V - FITC Conjugate, then 5 µl of PI Solution to the cell suspension.
- Incubate 15 minutes at room temperature with protection from light.
- Add 400 µl of 10-fold diluted Annexin V Binding Solution.
- Apply this solution to flow cytometric assay or microscopic assay.
*Although adherent cells are not frequently used for Annexin V, FITC flow cytometric analyses to avoid cell membrane damage from the cell detachment process, Casiola-Rosen et al. and van Engelend et al. have reported methods on utilizing Annexin V for flow cytometry with adherent cell types.
| excitation / emission | |
|---|---|
| Annexin V-FITC | 494 nm / 518 nm |
| PI | 535 nm / 617 nm |
References
- Casciola-Rosen L, Rosen A, Petri M, Schlissel M, Proc Natl Acad Sci USA, 93(4), 1624 (1996)
- van Engeland M, Remaekers FC, Schutte B, Reutelingsperger CP, Cytometry, 24(2), 131 (1996)
Downloads
Annexin V-FITC Apoptosis Detection Kit ![]() (PDF 3MB)
(PDF 3MB)
Ordering information
*1 test = 1 assay using 1x106 cells/ml solution
Related Product
| Product Name | Storage | PKG Size | Product No. | Price |
|---|---|---|---|---|
| Trypan Blue Solution | Room Temp. | 100 ml | 20577-34 | |
| MTT Cell Count Kit | -20°C | 1 kit | 23506-80 | |
| Cell Count Reagent SF | 4 °C | 500 tests | 07553-15 | |
| 2,500 tests | 07553-44 |
(Storage) RT: Room temperature, A: Cool and dark, R: Refrigerator, F: Freeze