
COSMOSIL Buckyprep
コスモシール Buckyprep は、フラーレン分離のスタンダードカラムです。特に高次フラーレンやフラーレン誘導体の分離に最適です。
特長
- フラーレン分離のスタンダード
- 誘導体化フラーレンや高次フラーレンの分離に最適
製品説明
物性
| 充填剤名称 | Buckyprep |
|---|---|
| シリカゲル | 全多孔性球状高純度シリカゲル |
| 平均粒子径(µm) | 5 |
| 平均細孔径(nm) | 12 |
| 比表面積(m2/g) | 300 |
| 固定相構造 | 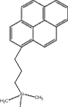 |
| 化学結合基 | ピレニルプロピル基 |
| USP カテゴリー | - |
| 結合形式 | モノメリック |
| 主な相互作用 | - |
| エンドキャッピング | あり |
| 炭素含有率(%) | 17 |
| 使用可能 pH 範囲 | 2 〜 7.5 |
製品使用例
分析例
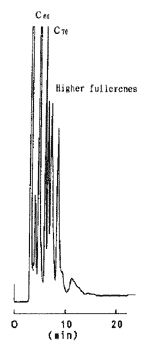
高次フラーレン
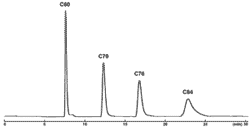
| Conditions | |||
|---|---|---|---|
| Column | COSMOSIL Buckyprep 4.6 mm I.D. × 250 mm | Detection | UV 312 nm |
| Mobilephase | Toluene | Sample | C60、C70、C76、C84 |
| Flow rate | 1.0 mL/min | ||
| Temperature | 30℃ | ||
酸化フラーレン
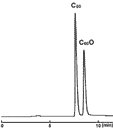
| Conditions | |||
|---|---|---|---|
| Column | COSMOSIL Buckyprep 4.6 mm I.D. × 250 mm | Detection | UV 312 nm |
| Mobilephase | Toluene | Sample | C60、 C60O |
| Flow rate | 1.0 mL/min | ||
| Temperature | 30℃ | ||
2 種類の移動相による高次フラーレンの分析
コスモシール Buckyprep カラムではフラーレンに対して強い保持力を示しますので、C70 以上の高次フラーレン(Higher Fullerenes)の溶出がトルエン移動相では困難になる場合があります。 トルエンよりも溶出力の強いクロロベンゼンを移動相として用いることによって、フラーレンの保持を約 1/3 にすることができます。
| 移動相 トルエン | 移動相 クロロベンゼン |
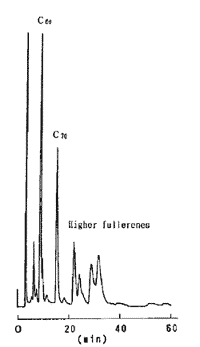 |
 |
| Conditions | |||
|---|---|---|---|
| Column | COSMOSIL Buckyprep 4.6 mm I.D. × 250 mm | Temperature | 30℃ |
| Flow rate | 1.0 mL/min | Detection | UV 285 nm |
使用文献
- Clikeman TT, Deng SHM, Popov AA, Wang XB, Strauss SH, Boltalina OV. Fullerene cyanation does not always increase electron affinity: an experimental and theoretical study. Phys. Chem. Chem. Phys. 2015;17:551-556.
- Ritter U, Prylutskyy YI, Evstigneev MP, Davidenko NA, Cherepanov VV, Senenko AI, Marchenko OA, Naumovets AG. Structural Features of Highly Stable Reproducible C60 Fullerene Aqueous Colloid Solution Probed by Various Techniques. Fullerenes, Nanotubes and Carbon Nanostructures. 2015;23 (6):530-534.
- Bortel G, Kovats E, Jakab E, Pekker S. Solvent-free Sc3N@C80-Ih and Its Precursor Cocrystal with Toluene. Fullerenes, Nanotubes and Carbon Nanostructures. 2015;23 (6):557-565.
- Abe Y, Matsuo Y. 1,8-Diazabicycloundecene-mediated Separation of Singly Bonded Fullerene Dimer and Application to Facile Preparation of C61H2. Fullerenes, Nanotubes and Carbon Nanostructures. 2015;23 (3):259-262.
- Apenova MG, Akhmetov VA, Belov NM, Goryunkov AA, Ioffe IN, Lukonina NS, Markov VY, Sidorov LN. Alkali-Metal Trichloroacetates for Dichloromethylenation of Fullerenes: Nucleophilic Addition-Substitution Route. Chemistry - An Asian Journal. 2014;9 (3):915-923.
- Wei T, Tamm NB, Yang S, Troyanov SI. New Trifluoromethylated Derivatives of Metal Nitride Clusterfullerenes: Sc3N@Ih-C80(CF3)14 and Sc3N@D5h-C80(CF3)16. Chemistry - An Asian Journal. 2014;9 (9):2449-2452.
- Belov NM, Apenova MG, Rybalchenko AV, Borkovskaya EV, Lukonina NS, Goryunkov AA, Ioffe IN, Troyanov SI, Sidorov LN. Transalkylation of Higher Trifluoromethylated Fullerenes with C70: A Pathway to New Addition Patterns of C70(CF3)8. Chemistry - A European Journal. 2014;20 (4):1126-1133.
- Carboni A, Emke E, Parsons JR, Kalbitz K, de Voogt P. An analytical method for determination of fullerenes and functionalized fullerenes in soils with high performance liquid chromatography and UV detection. Analytica Chimica Acta. 2014;807 (7):159-165.
- Tao R, Umeyama T, Kurotobi K, Imahori H. Effects of Alkyl Chain Length and Substituent Pattern of Fullerene Bis-Adducts on Film Structures and Photovoltaic Properties of Bulk Heterojunction Solar Cells. ACS Appl. Mater. Interfaces. 2014;6 (19):17313-17322.
- Larson BW, Whitaker JB, Popov AA, Kopidakis N, Rumbles G, Boltalina OV, Strauss SH. Thermal [6,6] → [6,6] Isomerization and Decomposition of PCBM (Phenyl-C61-butyric Acid Methyl Ester). Chem. Mater. 2014;26 (7):2361-2367.
- Xue JY, Ikemoto K, Takahashi N, Izumi T, Taka H, Kita H, Sato S, Isobe H. Cyclo-meta-phenylene Revisited: Nickel-Mediated Synthesis, Molecular Structures, and Device Applications. J. Org. Chem. 2014;79 (20):9735-9739.
- Matsuo Y, Ogumi K, Maruyama M, Nakagawa T. Divergent Synthesis and Tuning of the Electronic Structures of Cobalt-Dithiolene-Fullerene Complexes for Organic Solar Cells. Organometallics. 2014;33 (3):659-664.
- Karakawa M, Nagai T, Adachi K, Ie Y, Aso Y. N-phenyl[60]fulleropyrrolidines: alternative acceptor materials to PC61BM for high performance organic photovoltaic cells. J. Mater. Chem. A. 2014;2:20889-20895.
- Tuktarov AR, Khuzin AA, Natal'ya R. Popod'ko & Usein M. Dzhemilev. Synthesis and Tribological Properties of Sulfur-Containing Methanofullerenes. Fullerenes, Nanotubes and Carbon Nanostructures. 2014;22 (4):397-403.
- Liu C, Li Y, Chi D, Chen S, Liu T, Wang J, Liu H, Li Y. A Facile Way for Synthesis of High Performance Electron Receptor MCB: A Promising Replacer of PCBM. Fullerenes, Nanotubes and Carbon Nanostructures. 2014;22 (1-3):289-298.
- Bulgakov RG, Kinzyabaeva ZS. Synthesis of fullerene Γ-lactones by free radical reaction of C60 with ketones promoted by compounds of transition metals Cr(VI), Ce(IV). Russian Journal of Organic Chemistry. 2014;50 (8):1189-1193.
- A. S. Pimenova, A. A. Kozlov, A. A. Goryunkov, V. Yu. Markov, P. A. Khavrel, S. M. Avdoshenko, I. N. Ioffe, S. G. Sakharov, S. I. Troyanov and L. N. Sidorov. Synthesis and characterization of difluoromethylene-homo[60]fullerene, C60(CF2). Chem. Commun. 2007;374 - 376.
- Prylutska SV, Matyshevska OP, Grynyuk II, Prylutskyy YI, Ritter U, Scharff P. Biological Effects of C60 Fullerenes in vitro and in a Model System. Molecular Crystals and Liquid Crystals. 2007;468(1):2007, 265-274.
- Papina TS, Luk’yanova VA, Goryunkov AA, Ioffe IN, Gol’dt IV, Buyanovskaya AG, Kabaeva NM, Sidorov LN. The enthalpy of formation of fullerene fluoride C60F18 and the C-F bond energy. Russian Journal of Physical Chemistry A, Focus on Chemistry. 2007;81(10):1560-1564.
- Goryunkov AA, Dorozhkin EI, Tamm NB, Ignat’eva DV, Avdoshenko SM, Sidorov LN, Troyanov SI. Synthesis and molecular structure of 1,6,11,16,18,24,27,36-C60(CF3)8. Mendeleev Communications. 2007;17(2):110-112.
関連資料
取扱説明書 :
COSMOSIL Technical Note :
価格表
分析・分取カラム(粒子径 5 µm)
COSMOSIL Buckyprep
※価格表に記載のないカラムサイズをご要望の際はお問い合わせください。
COSMOSIL / コスモシールはナカライテスク株式会社の登録商標です。
※掲載内容は予告なく変更になる場合があります。


