COSMOGEL® GST-Accept
GST(グルタチオン S-トランスフェラーゼ)タグは、タンパク質のアフィニティー精製において His タグと並び使用頻度の高いエピトープタグです。GST がそのリガンドであるグルタチオンと特異的に結合することから、グルタチオンを担体へ連結することで効率的に GST タグ融合タンパク質を精製することができます。
- 優れた耐久性
- 優れたコストパフォーマンス
製品説明
製品仕様
| リガンド | グルタチオン |
|---|---|
| 粒子サイズ | 50 ~ 150 µm |
| マトリックス | 4% アガロース |
| 回収容量 | >5 mg/mL(回収量 / ゲル量) |
| 推奨線流速 | 26 cm/h |
| 限界圧 | 2.6 psi(0.18 bar) |
| 供給状態 | 50vol% 懸濁液(20vol% エタノール水溶液) |
薬品耐性
回収量に影響を及ぼさない添加物・緩衝液
| 還元剤 | 20 mmol/L DTT | 20 mmol/L 2-Mercaptoethanol |
|---|---|---|
| 20 mmol/L TCEP | ||
| 界面活性剤 | 1% Triton X-100 | 1% Tween 20 |
| 1% Nonidet P-40 | 1% CHAPS | |
| 1% Brij 35 | 1% 1-O-n-Octyl-β-D-glucopyranoside | |
| 1% n-Dodecyl-β-D-maltopyranoside | ||
| 添加剤 | 50 mmol/L EDTA | 50 mmol/L EGTA |
| 2 mol/L NaCl | 1 mol/L MgCl2 | |
| 500 mmol/L Imidazole | 20% Ethanol | |
| Protease Inhibitor Cocktail*1 (5×) | Phosphatase Inhibitor Cocktail*2 (5×) | |
| 緩衝液 | 100 mmol/L HEPES(pH 7.4, 8.0) | 100 mmol/L Tris-HCl(pH 7.4, 8.0) |
上記添加物を含む結合・溶出緩衝液を用いて確認したものであり、上限値を示すものではありません。また記載されていない添加物の使用を制限するものでもありません。融合している標的タンパク質によっては上記添加物の影響を受ける場合がありますので、あらかじめ小スケールで検討を行ってください。
*1 弊社 Protease Inhibitor Cocktail(100×)(一般用 : #04080、EDTA free : #03969)
*2 弊社 Phosphatase Inhibitor Cocktail(100×)(#07574-61、EDTA free : #07575-51)
Triton はユニオン・カーバイド・コーポレーション、Tween はクローダ インターナショナル ピーエルシー、Nonidet はシェル ブランズ インターナショナル アクチェンゲゼルシャフト、Brij はクローダ アメリカス リミテッド ライアビリティ カンパニーの登録商標です。
性能評価
GST タグ融合タンパク質の 10 回連続精製
GST-His を発現させた大腸菌ライセートを同一のゲルを用いて 10 回連続して精製しました。10 回目でも問題なく精製できています。
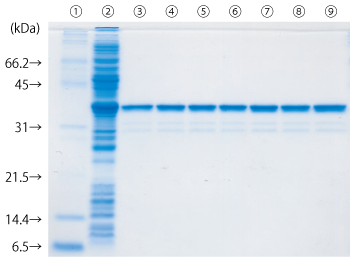
Lane
- タンパク質マーカー(10倍濃縮)(#29458-24)を 1 × で使用
- 大腸菌ライセート
- 1 回目の溶出画分
- 2 回目の溶出画分
- 3 回目の溶出画分
- 4 回目の溶出画分
- 6 回目の溶出画分
- 8 回目の溶出画分
- 10 回目の溶出画分
- タンパク質マーカー(10倍濃縮)(#29458-24)を 1 × で使用
サンプル : E. coli(BL21(DE3)pLysS) lysate transformed with GST-His(pET-41b(+))
結合緩衝液 : Phosphate Buffered Saline(10x)(pH 7.4)(#27575-31)を 1 × で使用
溶出緩衝液 : 10 mM Glutathione, 50 mM Tris-HCl, pH 8.0
製品使用例
GST タグ融合タンパク質の Thrombin によるカラム内消化
Thrombin(#33842-44)無添加の場合は発現タンパク質がそのまま回収されていますが、Thrombin 添加の場合は PBS 画分(Lane7)に S タグ - His タグが溶出しています。このことから、カラム内消化も問題なく行えていることがわかります。
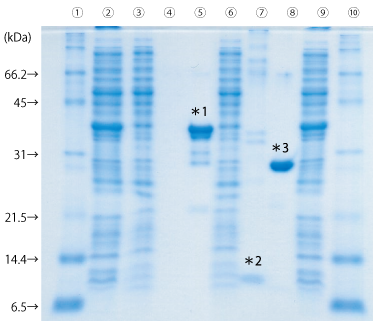
Lane
- タンパク質マーカー(10倍濃縮)(#29458-24)を 1 × で使用
- 大腸菌ライセート
- フロースルー画分
- Thrombin (-) PBS fraction
- Thrombin (-) glutathione fraction
- フロースルー画分
- Thrombin (+) PBS fraction
- Thrombin (+) glutathione fraction
- 大腸菌ライセート
- タンパク質マーカー(10倍濃縮)(#29458-24)を 1 × で使用
*1 GST タグ - His タグ - S タグ - His タグ
*2 S タグ - His タグ
*3 GST タグ - His タグ
サンプル : E. coli(BL21(DE3)pLysS) lysate transformed with GST-His(pET-41b(+))
結合緩衝液 : Phosphate Buffered Saline(10x)(pH 7.4)(#27575-31)を 1 × で使用
溶出緩衝液 : 10 mM Glutathione, 50 mM Tris-HCl, pH 8.0
使用文献
- Liu XQ, Zhang D, Zhang XM, Wang CT, Liu XQ, Tan YP, Wu YH. Study on the interaction between methyl jasmonate and the coiled-coil domain of rice blast resistance protein Pi36 by spectroscopic methods. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2012;88:72-76.
- Tanio M, Nishimura K. Analysis of the phospholipase C-δ1 pleckstrin homology domain using native polyacrylamide gel electrophoresis. Analytical Biochemistry. 2012;431(2):106-114.
- Nagae S, Meng W, Takeichi M. Non-centrosomal microtubules regulate F-actin organization through the suppression of GEF-H1 activity. Genes to Cells. 2013;18(5):387-396.
- Takino J, Nagamine K, Hori T. Ras guanyl nucleotide releasing protein 2 affects cell viability and cell-matrix adhesion in ECV304 endothelial cells. Cell Adhesion & Migration. 2013;7(3):262-266.
- Inoue H, Hayashi N, Matsushita A, Liu X, Nakayama A, Sugano S, Jiang CJ, Takatsuji H. Blast resistance of CC-NB-LRR protein Pb1 is mediated by WRKY45 through protein–protein interaction. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(23):9577-9582.
- Tokunaga M, Shiheido H, Tabata N, Sakuma-Yonemura Y, Takashima H, Horisawa K, Doi N, Yanagawa H. MIP-2A Is a Novel Target of an Anilinoquinazoline Derivative for Inhibition of Tumour Cell Proliferation. PLOS ONE. 2013;8(9):e76774. https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0076774&type=printable, (cited 2020-02-10)
- Oda T, Yagi T, Yanagisawa H, Kikkawa M. Identification of the Outer-Inner Dynein Linker as a Hub Controller for Axonemal Dynein Activities. Current Biology. 2013;23(8):656-664.
- Fujioka Y, Suzuki SW, Yamamoto H, Kondo-Kakuta C, Kimura Y, Hirano H, Akada R, Inagaki F, Ohsumi Y, Noda NN. Structural basis of starvation-induced assembly of the autophagy initiation complex. Nature Structural & Molecular Biology. 2014;21:513-521.
- Fujiwara Y, Goda N, Tamashiro T, Narita H, Satomura K, Tenno T, Nakagawa A, Oda M, Suzuki M, Sakisaka T, Takai Y, Hiroaki H. Crystal structure of afadin PDZ domain–nectin-3 complex shows the structural plasticity of the ligand-binding site. PROTEIN SCIENCE. 2015;24(3):376-385.
- Suzuki K, Sako K, Akiyama K, Isoda M, Senoo C, Nakajo N, Sagata N. Identification of non-Ser/Thr-Pro consensus motifs for Cdk1 and their roles in mitotic regulation of C2H2 zinc finger proteins and Ect2. Scientific Reports. 2015;5:7929. https://www.nature.com/articles/srep07929, (cited 2020-02-10)
- Kurogochi M, Mori M, Osumi K, Tojino M, Sugawara S, Takashima S, Hirose Y, Tsukimura W, Mizuno M, Amano J, Matsuda A, Tomita M, Takayanagi A, Shoda S, Shirai T. Glycoengineered Monoclonal Antibodies with Homogeneous Glycan (M3, G0, G2, and A2) Using a Chemoenzymatic Approach Have Different Affinities for FcγRIIIa and Variable Antibody-Dependent Cellular Cytotoxicity Activities. PLOS ONE. 2015;10(7):e0132848. https://journals.plos.org/plosone/article/authors?id=10.1371/journal.pone.0132848, (cited 2020-02-10)
- Abiko Y, Ishii I, Kamata S, Tsuchiya Y, Watanabe Y, Ihara Hideshi, Akaike T, Kumagai Y. Formation of Sulfur Adducts of N-Acetyl-p-benzoquinoneimine, an Electrophilic Metabolite of Acetaminophen in Vivo: Participation of Reactive Persulfides. Chemical Research in Toxicology. 2015;28(9):1796-1802.
- Liu X, Inoue H, Hayashi N, Jiang CJ, Takatsuji H. CC-NBS-LRR-Type R Proteins for Rice Blast Commonly Interact with Specific WRKY Transcription Factors. Plant Molecular Biology Reporter. 2016;34(2):533-537.
- Yang X, Matsui T, Mori T, Taura F, Noguchi H, Abe I, Morita H. Expression, purification and crystallization of a plant polyketide cyclase from Cannabis sativa. Acta Crystallographica Section F. 2015;71(12):1470-1474.
- Shirai T, Mori M, Kurogochi M, Tomita M. METHOD FOR PREPARING GLYCAN-HYDROLYZED ANTIBODY, AND HOMOGENEOUS GLYCOSYLATED ANTIBODY. United Sates Patent Application. 20160032010.
- Matsumoto D, Tao R. Recognition of a wide-range of S-RNases by S locus F-box like 2, a general-inhibitor candidate in the Prunus-specific S-RNase-based self-incompatibility system. Plant Molecular Biology. 2016;91(4-5):459-469.
- Kii I, Sumida Y, Goto T, Sonamoto R, Okuno Y, Yoshida S, Kato-Sumida T, Koike Y, Abe M, Nonaka Y, Ikura T, Ito N, Shibuya H, Hosoya T, Hagiwara M. Selective inhibition of the kinase DYRK1A by targeting its folding process. Nature Communications. 2016;7:11391. https://www.nature.com/articles/ncomms11391?WT.ec_id=NCOMMS-20160423, (cited 2020-02-10)
- Yamasaki A, Watanabe Y, Adachi W, Suzuki K, Matoba K, Kirisako H, Kumeta H, Nakatogawa H, Ohsumi Y, Inagaki F, Noda NN. Structural Basis for Receptor-Mediated Selective Autophagy of Aminopeptidase I Aggregates. Cell Reports. 2016;16(1):19-27.
- Yamamoto H, Fujioka Y, Suzuki SW, Noshiro D, Suzuki H, Kondo-Kakuta, C, Kimura Y, Hirano H, Ando T, Noda NN, Ohsumi Y. The Intrinsically Disordered Protein Atg13 Mediates Supramolecular Assembly of Autophagy Initiation Complexes. Developmental Cell. 2016;38(1):86-99.
- Miura Y, Hongu T, Yamauchi Y, Funakoshi Y, Katagiri N, Ohbayashi N, Kanaho Y. ACAP3 regulates neurite outgrowth through its GAP activity specific to Arf6 in mouse hippocampal neurons. Biochemical Jouranl. 2016;473(17):2591-2602.
- Hanaoka K, Sasakura K, Suwanai Y, Toma-Fukai S, Shimamoto K, Takano Y, Shibuya N, Terai T, Komatsu T, Ueno T, Ogasawara Y, Tsuchiya Y, Watanabe Y, Kimura H, Wang C, Uchiyama M, Kojima H, Okabe T, Urano Y, Shimizu T, Nagano T. Discovery and Mechanistic Characterization of Selective Inhibitors of H2S-producing Enzyme: 3-Mercaptopyruvate Sulfurtransferase (3MST) Targeting Active-site Cysteine Persulfide. Scientific Reports. 2017;7:40227. https://www.nature.com/articles/srep40227, (cited 2020-02-10)
- Shimasaki T, Ohtsuka H, Naito C, Azuma K, Tenno T, Hiroaki H, Murakami H, Aiba H. Ecl1 is a zinc-binding protein involved in the zinc-limitation-dependent extension of chronological life span in fission yeast. Molecular Genetics and Genomics. 2017;292(2):475-481.
- Inoshita M, Mima J. Human Rab small GTPase– and class V myosin–mediated membrane tethering in a chemically defined reconstitution system. The Journal of Biological Chemistry. 2017;292(45):18500-18517.
- Uchida T, Kobayashi N, Muneta S, Ishimori K. The Iron Chaperone Protein CyaY from Vibrio cholerae Is a Heme-Binding Protein. Biochemistry. 2017;56(18):2425-2434.
- Tsukimura W, Kurogochi M, Mori M, Osumi K, Matsuda A, Takegawa K, Furukawa K, Shirai T. Preparation and biological activities of anti-HER2 monoclonal antibodies with fully core-fucosylated homogeneous bi-antennary complex-type glycans. Bioscience, Biotechnology, and Biochemistry. 2017;81(12):2353-2359.
- Suzuki H, Noda NN. Biophysical characterization of Atg11, a scaffold protein essential for selective autophagy in yeast. FEBS openbio. 2018;8(1):110-116.
- Kusunoki H, Tanaka T, Kohno T, Matsuhashi K, Hosoda K, Wakamatsu K, Hamaguchi I. A novel neuropilin-1–binding sequence in the human T-cell lymphotropic virus type 1 envelope glycoprotein. Bochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2018;1866(4):541-548.
- Hori K, Ajioka K, Goda N, Shindo A, Takagishi M, Tenno T, Hiroaki H. Discovery of Potent Disheveled/Dvl Inhibitors Using Virtual Screening Optimized With NMR-Based Docking Performance Index. Frontiers in Pharmacology. 2018;9:983. https://www.frontiersin.org/articles/10.3389/fphar.2018.00983/full, (cited 2020-02-10)
- Okazaki H. Matsuo N, Tenno T, Goda N, Shigemitsu Y, Ota M, Hiroaki H. Using 1HN amide temperature coefficients to define intrinsically disordered regions: An alternative NMR method. PROTEIN SCIENCE. 2018;27(10):1821-1830.
- Senga S, Kawaguchi K, Kobayashi N, Ando A, Fujii H. A novel fatty acid-binding protein 5-estrogen-related receptor α signaling pathway promotes cell growth and energy metabolism in prostate cancer cells. Oncotarget. 2018;9(60):31753-31770.
- Ueno S, Seno K, Maruyama Y, Hayashi F, Miyoshi H, Morita M, Maekawa S. Lipid Components in the Dynamin Fraction Prepared from Rat Brain. International Journal of Lipids. 2018;1(1):1-10. https://openaccesspub.org/ijl/article/763, (cited 2020-02-10)
- Liu XM, Yamasaki A, Du XM, Coffman VC, Ohsumi Y, Nakatogawa H, Wu JQ, Noda NN, Du LL. Lipidation-independent vacuolar functions of Atg8 rely on its noncanonical interaction with a vacuole membrane protein. eLife, 2018;7:e41237. https://elifesciences.org/articles/41237, (cited 2020-02-10)
- Yamasaki A, Watanabe Y, Noda NN. Structural Studies of Selective Autophagy in Yeast. Autophagy (Part of the Methods in Molecular Biology book series) . 2019:77-90.
- Segawa K, Tamura N, Mima J. Homotypic and heterotypic trans-assembly of human Rab-family small GTPases in reconstituted membrane tethering. The Journal of Biological Chemistry. 2019;294:7722-7739.
- Matsumoto D, Tao R. Recognition of S-RNases by an S locus F-box like protein and an S haplotype-specific F-box like protein in the Prunus-specific self-incompatibility system. Plant Molecular Biology. 2019;100(4-5):367-378.
- Kusamoto H, Kinoshita-Kikuta E, Nishimura T, Nagai T, Kinoshita E, Koike T. Gel-based analysis of protein phosphorylation status by rapid fluorometric staining using TAMRA-labeled Phos-tag. Journal of Electrophoresis. 2019;63(1):25-32.
- Okuda J, Nagata S, Yasuda M, Suezawa C. Validating the inhibitory effects of D- and L-serine on the enzyme activity of D-3-phosphoglycerate dehydrogenases that are purified from Pseudomonas aeruginosa, Escherichia coli and human colon. Gut Pathogens. 2019;11:35. https://doi.org/10.1186/s13099-019-0315-8, (cited 2020-02-10)
- Saneyoshi T, Matsuno H, Suzuki A, Murakoshi H, Hedrick NG, Agnello E, O'Connell R, Stratton MM, Yasuda R, Hayashi Y. Reciprocal Activation within a Kinase-Effector Complex Underlying Persistence of Structural LTP. Neuron. 2019;102(6):1199-1210.
- Kojima H, Rosendale M, Sugiyama Y, Hayashi M, Horiguchi Y, Yoshihara T, Ikegaya Y, Saneyoshi T, Hayashi Y. The role of CaMKII-Tiam1 complex on learning and memory. Neurobiology of Learning and Memory. 2019;166:107070. https://www.sciencedirect.com/science/article/pii/S1074742719301376?via%3Dihub, (cited 2020-02-10)
- 廣明秀一. 構造情報を利用したタイトジャンクション増強剤の実用的な化合物への骨格転換. コスメトロジー研究報告. 2019, 27, p. 137-144.
- Yamanishi K, Fiedler M, Terawaki S, Higuchi Y, Bienz M, Shibata N. A direct heterotypic interaction between the DIX domains of Dishevelled and Axin mediates signaling to β-catenin. Science Signaling. 2019;12(611):eaaw5505. https://stke.sciencemag.org/content/12/611/eaaw5505.abstract, (cited 2020-02-10)
- Yamasaki A, Noda NN. Structural study on Atg8-receptor complexes that mediate ER-phagy. Photon Factory Activity Report 2019. 2020;37:BL-1A, AR-NE3A/2019RP-19.
- Fujioka Y, Md. Alam J, Noshiro D, Mouri K, Ando T, Okada Y, I. May A, L. Knorr R, Suzuki K, Ohsumi Y, Noda NN. Phase separation organizes the site of autophagosome formation. Nature. 2020;578:301-305.
- Yamasaki A, Md. Alam J, Noshiro D, Hirata E, Fujioka Y, Suzuki K, Ohsumi Y, Noda NN. Liquidity Is a Critical Determinant for Selective Autophagy of Protein Condensates. Molecular Cell. 2020;77(6):1163-1175.
- Taniguchi S, Toyoshima M, Takamatsu T, Mima J. Curvature‐sensitive trans‐assembly of human Atg8‐family proteins in autophagy‐related membrane tethering. Protein Science. 2020;29(6):1387-1400.
- Mochida K, Yamasaki A, Matoba K, Kirisako H, Noda NN, Nakatogawa H. Super-assembly of ER-phagy receptor Atg40 induces local ER remodeling at contacts with forming autophagosomal membranes. Nature Communications. 2020;11:3306.
- Ueda S, Tamura N, Mima J. Membrane Tethering Potency of Rab-Family Small GTPases Is Defined by the C-Terminal Hypervariable Regions. Frontiers in Cell and Developmental Biology. 2020;8:577342.
- Nakashima M, Hisada M, Goda N, Tenno T, Kotake A, Inotsume Y, Kameoka I, Hiroaki H. Opposing Effect of Naringenin and Quercetin on the Junctional Compartment of MDCK II Cells to Modulate the Tight Junction. Nutrients. 2020;12(11):3285.
価格表
COSMOGEL コスモゲルはナカライテスク株式会社の登録商標です。
※掲載内容は予告なく変更になる場合があります。