ACROBiosystems社 Solutions for COVID-19 vaccine safety and immunogenicity evaluation
|
※最新情報はメーカーHPをご確認ください。 |
| Solutions for COVID-19 vaccine safety and immunogenicity evaluation |
|
Researchers around the world are working hard on a vaccine for COVID-19. Up until now, several vaccine candidates have entered into phase III clinical trials. Experts estimate that a fast-tracked vaccine development process could potentially bring a successful candidate to market within one year or even less. In the development of a COVID-19 vaccine, safety and immunogenicity evaluation are indispensable and well-established assays are required for fast and convenient assessment of the Based on advanced technology platform in recombinant protein production, ACROBiosystems has developed a series of critical reagents for COVID-19 vaccine development and evaluation, including recombinant proteins, antibodies and ELISA kits. These featured products are suitable for IgG/M antibody titer detection, neutralizing antibody titer detection and antigen titer detection to accelerate the vaccine development. |
|
Application >>> Scenario 1: IgG antibody titer detection >> Recommended products Cat.No. SPN-C52H9 Products: SARS-CoV-2 S protein, His Tag, Super stable trimer (MALS & NS-EM verified)
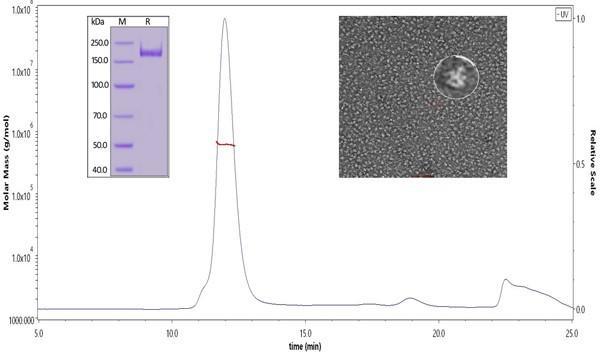
Fig 1. The purity of SARS-CoV-2 S protein, His Tag, Super stable trimer (Cat. No. SPN-C52H9) was more than 90% verified by SDS-PAGE under reducing (R) condition. The molecular weight was around 550-660 kDa confirmed by SEC-MALS. The particles are similar in size and appearance to SARS-CoV-2 trimers reported in published literature verified by negative stain electron micrography.
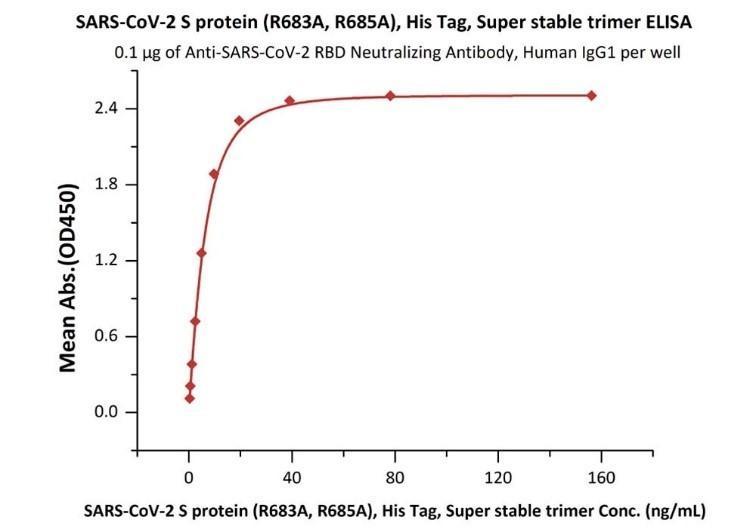
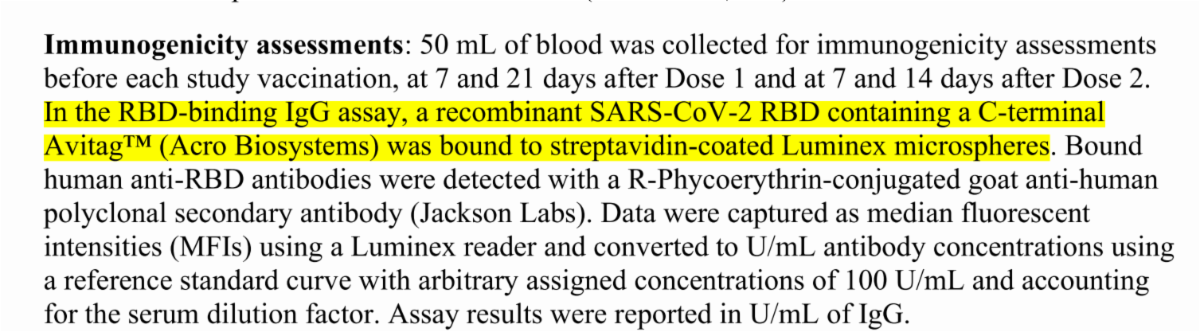
Fig 2. Immobilized Anti-SARS-CoV-2 Neutralizing Antibody, Human IgG1 (Cat. No. SAD-S35) at 1 μg/mL (100 μL/well) can bind SARS-CoV-2 S
Cat.No. SPD-C82E9 Products: Biotinylated SARS-CoV-2 (COVID-19) S protein RBD, His,Avitag™ (MALS verified)
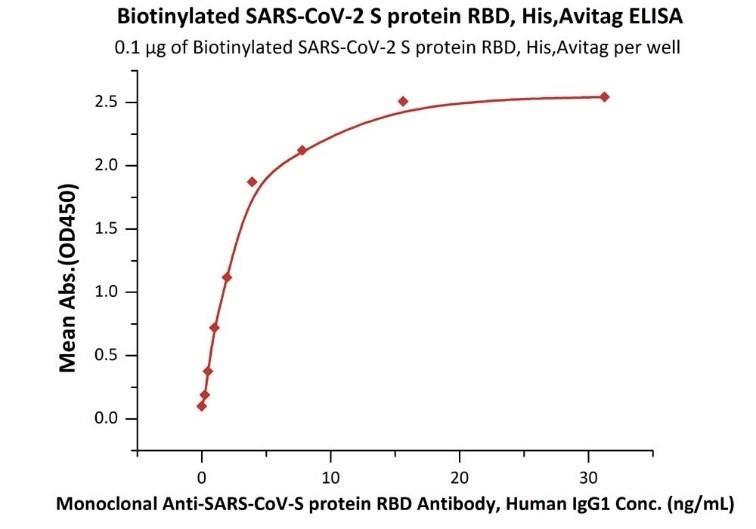
Fig 3. Immobilized Biotinylated SARS-CoV-2 S protein RBD, His,Avitag (Cat. No. SPD-C82E9) at 1 μg/mL (100 μL/well) on Streptavidin (Cat. No. STN-N5116) precoated (0.5 μg/well) plate, can bind Monoclonal Anti-SARS-CoV-S protein RBD Antibody, Human IgG1 with a linear range of 0.1-4 ng/mL
>> Case Study: Pfizer employed ACROBiosystems’ featured biotinylated S protein RBD in the RBD-binding IgG assay during the phase 1/2 research. The interim report showed that the biotinylated S protein RBD from ACROBiosystems (Cat.No. SPD-C82E9) was bound to streptavidin-coated Luminex microspheres.
Reference: Mulligan et al., (2020). Phase 1/2 Study to Describe the Safety and Immunogenicity of a COVID-19 RNA Vaccine Candidate (BNT162b1) in Adults 18 to 55 Years of Age: Interim Report. medRxiv, doi: http://life.acrobiosystems.cn/e/535472/10-1101-2020-06-30-20142570/2ltqp9b/1014937997?h=M2FjPthwmC8fSXvWbZNZx4pdEqIobujmrimSQq8Kg2Y
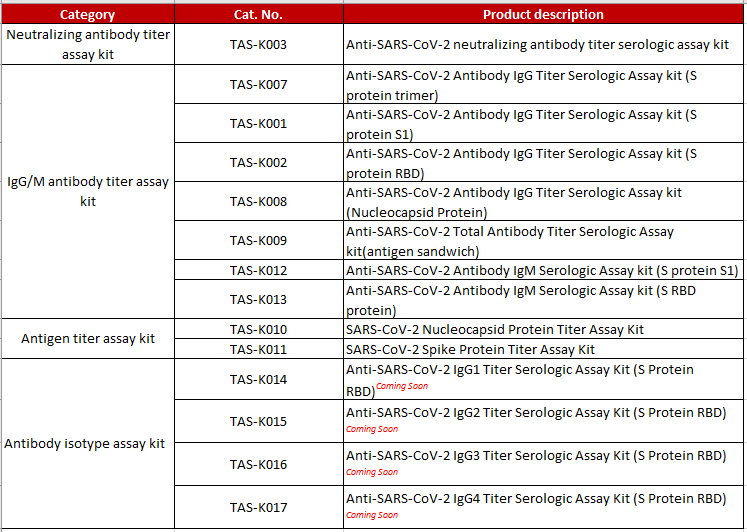
Cat.No. TAS-K002 Products: Anti-SARS-CoV-2 Antibody IgG Titer Serologic Assay kit (Spike protein RBD)
Fig 4. Immobilized SARS-CoV-2 S protein RBD at 2 μg/mL (100 μL/well) can bind Monoclonal Anti-SARS-CoV-2 Antibody, Human IgG1 in 1:50 human serum. Detection was performed using HRP-Conjugated Anti-human IgG antibody with sensitivity of 24 ng/mL |
|
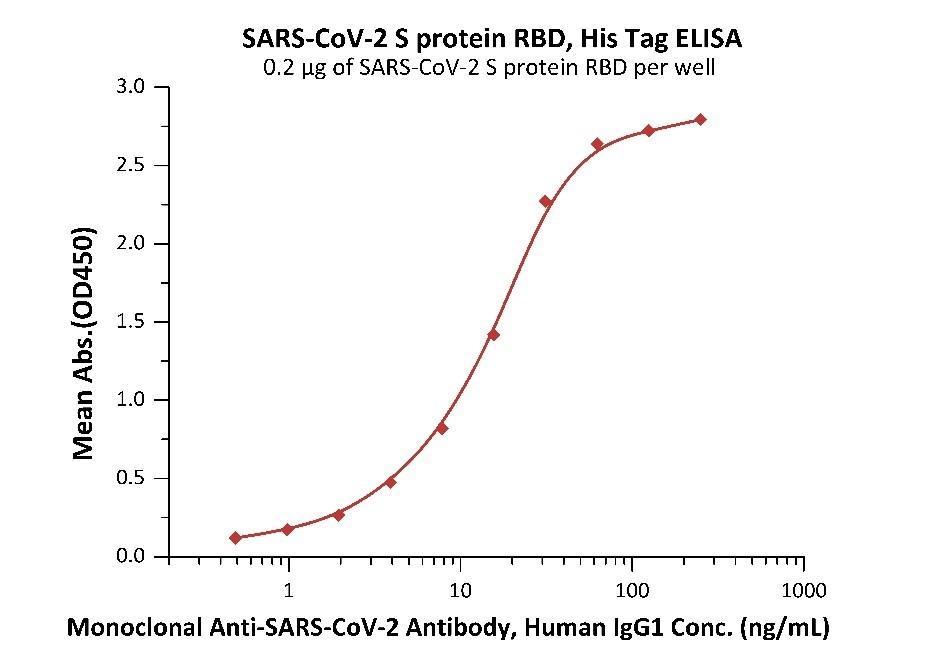
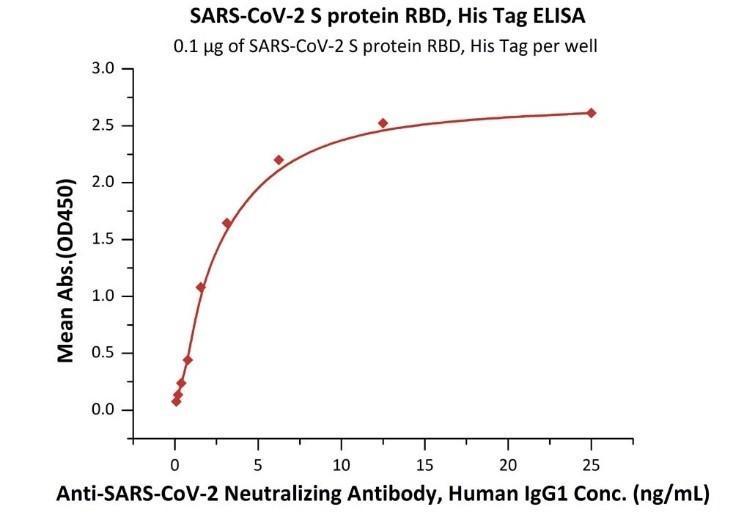
>>> Scenario 2: Neutralizing antibody titer detection >> Recommended products Cat.No. SPD-C52H3 Products:SARS-CoV-2 (COVID-19) S protein RBD, His Tag (MALS verified)
Fig 5. Immobilized SARS-CoV-2 S protein RBD, His Tag (Cat. No. SPD-C52H3) at 1 μg/mL (100 μL/well) can bind Anti-SARS-CoV-2 RBD Neutralizing Antibody, Human IgG1 (Cat. No. SAD-S35) with a linear range of 0.1-3 ng/mL
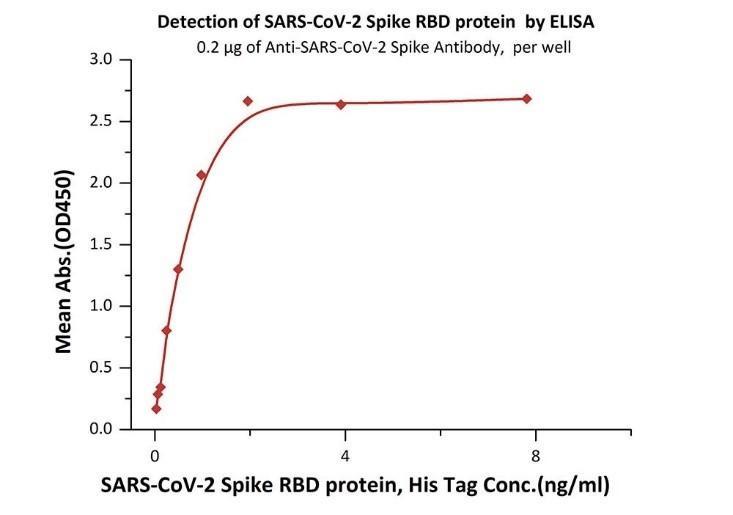
Fig 6. Immobilized SARS-CoV-2 S protein RBD, His Tag (Cat. No. SPD-C52H3) at 1 μg/mL (100 μL/well) can bind Human ACE2, Fc Tag (Cat. No. AC2-H5257) with a linear range of 0.2-3 ng/mL
Cat.No. TAS-K003 Products: Anti-SARS-CoV-2 Neutralizing Antibody Titer Serologic Assay kit
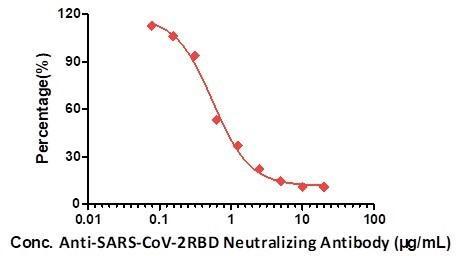
Fig 7. Neutralizing titer of Anti-SARS-CoV-2 Neutralizing Antibody, Human IgG1 (Cat.No.SAD-S35) measured by Anti-SARS-CoV-2 neutralizing antibody titer serologic assay kit (Cat. No. TAS-K003). |
|
>>> Scenario 3: Antigen titer detection >> Recommended products Cat.No. TAS-K011 Products: SARS-CoV-2 Spike Protein Titer Assay Kit
Fig 8. Immobilized Anti-SARS-CoV-2 Spike S1 Antibody at 2 μg/mL (100 μL/well) can bind SARS-CoV-2 Spike Protein. And then add Biotinylated Anti-SARS-CoV-2 Spike S1 Antibody at 1ug/ml. Detection was performed using HRP-conjugated streptavidin with sensitivity of 24 pg/mL |
|
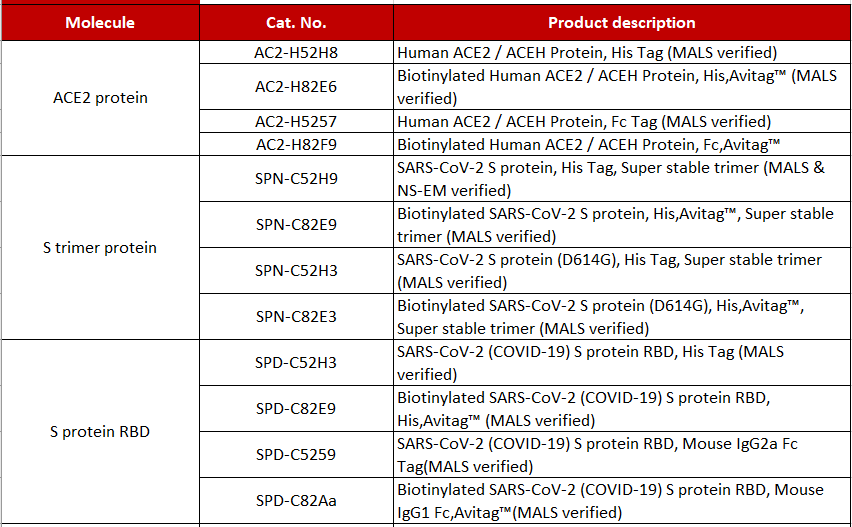
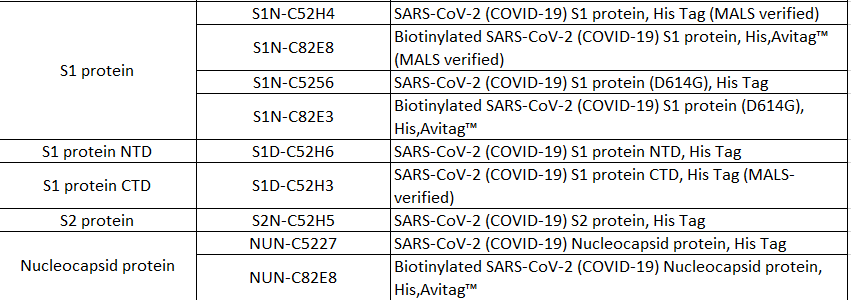
Product List >> Proteins
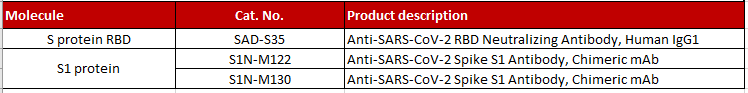
>> Antibodies
>> Kits |
Fount of Information は、新商品、新規取扱メーカーなどの情報をいち早く紹介するコンテンツです。情報発信のスピードを重視しているコンテンツのため、現時点で法規制や取り扱いを確認できていない商品、定価を設定できていない商品があります。ご要望やご照会を受けた商品について、法令整備や在庫の充実を図ります。