ACROBiosystems社 Insights:Facilitating Bispecific Antibody Drug Development through Quality Control
Bispecific antibodies(bsAbs) are a relatively new type of drug in the field of targeted therapeutics. By binding to both CD3 and a tumor-associated antigen, these bsAbs bridge T cells and tumor cells together to initiate tumor cell death, irrespective of T cell receptor specificity, co-stimulation, or peptide antigen presentation. Furthermore, the activation of T cells by CD3 bsAbs stimulates the secretion of a wide variety of cytokines to recruit immune helper cells to further enhance the anti-tumor cell effects. |
|
|
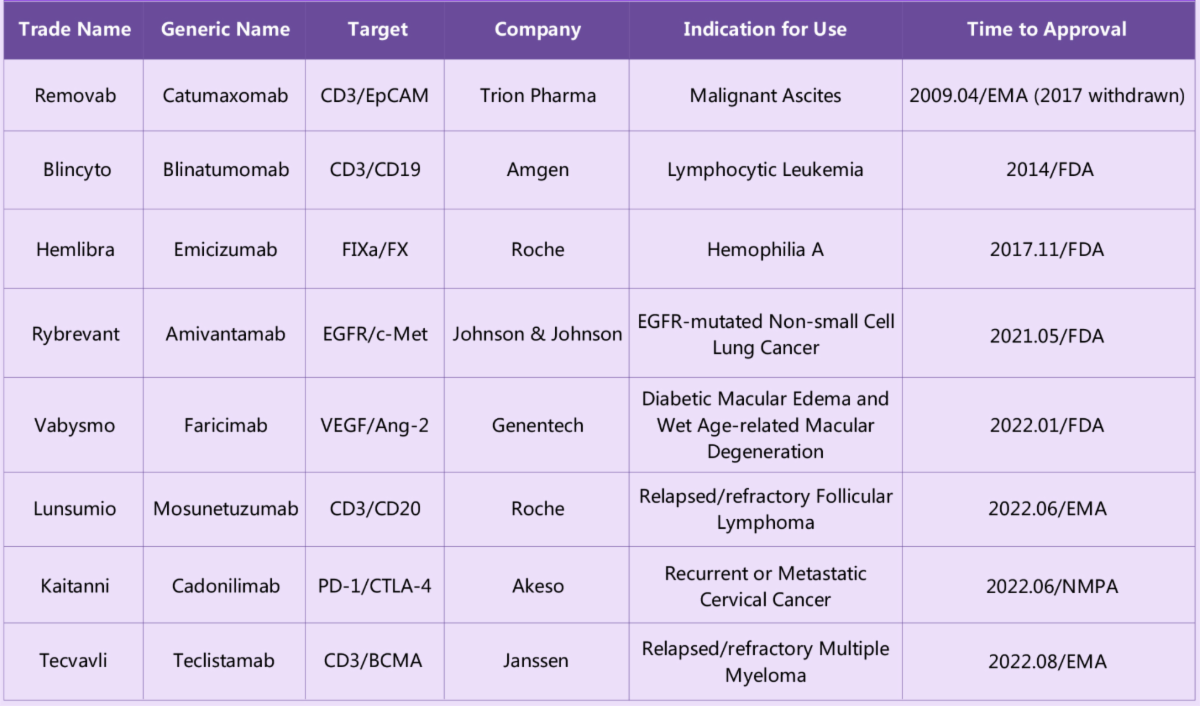
Current marketed bsAbs that have successfully received market approval and their listed indication of use
|
|
|
From the concept phase to clinical trials, many companies around the world are accelerating their research and development processes of these drugs, especially CD3-targeting bsAbs. This means that quality control of bsAb drugs becomes even more critical, especially as a novel immunotherapeutic. A key reagent for these quality control studies are recombinant proteins. To support bsAb research and its relevant quality control studies, ACROBiosystems strives to provide high-quality, bioactive, and consistent recombinant proteins including CD3 and many others.
|
Fount of Information は、新商品、新規取扱メーカーなどの情報をいち早く紹介するコンテンツです。情報発信のスピードを重視しているコンテンツのため、現時点で法規制や取り扱いを確認できていない商品、定価を設定できていない商品があります。ご要望やご照会を受けた商品について、法令整備や在庫の充実を図ります。