Creative Diagnostics社 Advanced Development of Antibody-Drug Conjugate (ADC) Immunoassay at Creative Diagnostics
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Immunoassay of Antibody-Drug Conjugates (ADCs) —Pharmacokinetic (PK) Characterization of ADCs |
|||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As a leader in the development of antibody-drug conjugates (ADCs), Creative Diagnostics has developed a portfolio of ELISA kits for the quantitative detection of conjugate antibodies targeting commonly encountered payloads such as MMAE, SN38 and DM1. These kits provide highly sensitive monitoring of conjugate antibody concentrations in serum and plasma, with high reproducibility and inter-batch consistency of test results. They are excellent tools for PK studies of ADCs.
For conjugated antibody quantification, the capture reagent is the antibody targeting the cytotoxic drug. After capturing the conjugated antibody, the amount is quantified using biotin antigen or anti-CDR mAb.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Key Features:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Performance Characteristics The Intact MMAE ADC ELISA Kit (DEIABL314) developed by Creative Diagnostics has demonstrated excellent validation data as shown in the table below:
Precision
Recovery
Analytical sensitivity
Standard Curve
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Product List
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
Fount of Information は、新商品、新規取扱メーカーなどの情報をいち早く紹介するコンテンツです。情報発信のスピードを重視しているコンテンツのため、現時点で法規制や取り扱いを確認できていない商品、定価を設定できていない商品があります。ご要望やご照会を受けた商品について、法令整備や在庫の充実を図ります。


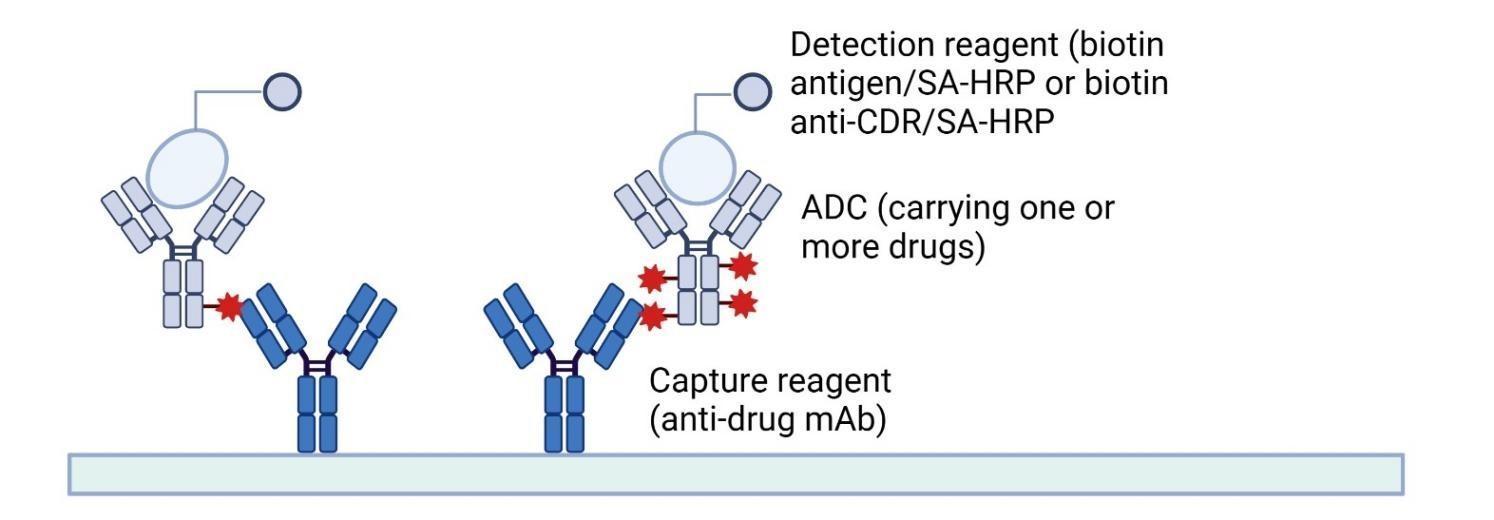 Fig.1 Principle of Conjugated Antibody Quantitative Detection
Fig.1 Principle of Conjugated Antibody Quantitative Detection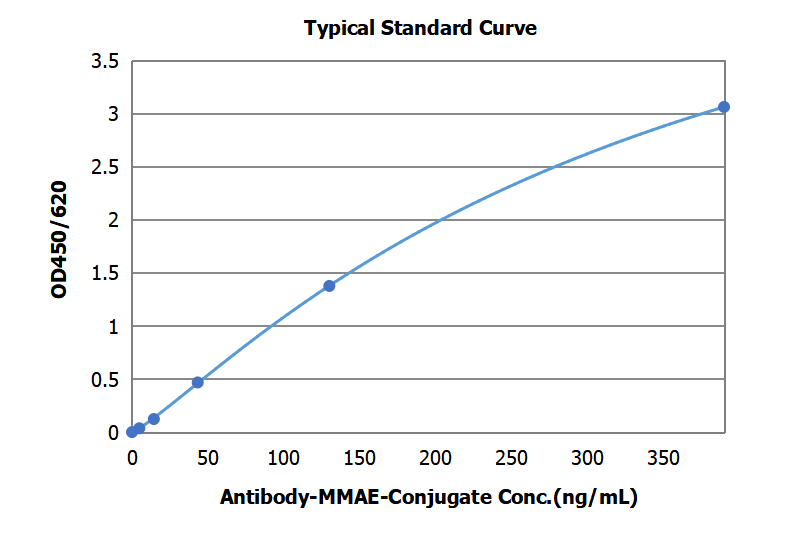 Fig.2 Typical Standard Curve of MMAE ADC ELISA Kit (DEIABL314)
Fig.2 Typical Standard Curve of MMAE ADC ELISA Kit (DEIABL314)