TriLink BioTechnologies社 August TriLink Messenger
|
この製品に関するご照会・お問合せはこちら


RESEARCH NEWS
|

| Mobile “On-Demand” Manufacturing of mRNA Vaccines |
|
Vaccines are typically manufactured in centralized facilities with large-scale production and purification capacities, requiring large capital expenditures and long lead times for construction and process development. This month's blog highlights mobile “on-demand” manufacturing of mRNA vaccines.

|

|
A long-acting form of Interleukin-7 improves CAR T cell activity
|
|
|
Mobilization could replace chemotherapy during treatment with gene-edited stem cells
|
|
|
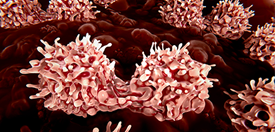
Co-treatment with a long-acting form of IL-7 and CAR T cell therapy in mouse models promises improved outcomes for patients with B cell malignancies. Learn how TriLink’s custom-made gRNA and Cas9 mRNA contributed to this discovery in our latest research spotlight.
|
Mobilization promises to replace chemotherapy as a means of bone marrow depletion, improving the lives of patients undergoing hematopoietic stem/progenitor cell gene therapy (HSPC-GT). Find out more in our recent research spotlight.
|
 |
 |
UPCOMING EVENTS
|

| Challenges and Considerations for mRNA Therapeutic Development |
This is the second webinar in a series of four, cohosted by GEN and The CRISPR Journal, that is diving deep into the burgeoning world of mRNA research and therapeutics. Our distinguished guest for this event, Dr. Edward Miracco, will take us through his extensive experience with mRNA chemistry, sequence design, and commercial-scale production. Dr. Miracco has faced many challenges in his work and will tell us about several vital considerations that could significantly aid your mRNA research endeavors.
Tuesday August 23, 2022, 11 a.m. ET
Wednesday August 24, 2022, 0 a.m. JST
 |
|
Fount of Information は、新商品、新規取扱メーカーなどの情報をいち早く紹介するコンテンツです。情報発信のスピードを重視しているコンテンツのため、現時点で法規制や取り扱いを確認できていない商品、定価を設定できていない商品があります。ご要望やご照会を受けた商品について、法令整備や在庫の充実を図ります。
