GERBU Adjuvant (Gerbu)
In 1991 Dr. Nikolaus Grubhofer started research to make his vision come true. He decided to develop a better adjuvant, which meant: match Freund's adjuvant as the yardstick of efficiency, make it perfectly biotolerable, remove the emulsion handling problem, maintain a reasonable price level and get it patented and licensed. Many data and formulations have become available as the result of the 50 years of scientific work following Freund1. The trouble with all this work was that it was done nearly exclusively with mice and was of very limited use with larger animals, which were the necessary models for veterinary and human vaccination.
In 2001 the GERBU Adjuvant (GAD) formulations basically were ready2: A microsuspension of cationized lipid solid particles in water combined with GMDP and other synergists. They could be simply mixed with the antigen solution and were as effective as Freund's FCA, also in other species such as sheep, goats, cattle and humans - but devoid of its drawbacks: They were completely biocompatible and easy to use, suitable for a wide range of applications3,4,5 and potentially for licensing in pharmaceutical use. After more than 10 years of development and field tests, GAD have emerged as the only efficient, biocompatible, convenient and economical successors of adjuvants as Freund's, Montanide or Alumina.
GERBU ADJUVANT - A powerful potentiator of cellular and humoral immune response with long-term protecting effect. Very stable and biocompatible. Just mix with antigen and inject.

- Efficiency even better than Complete Freunds Adjuvant
- Stability in long time storage at various temperatures and conditions
- Compatibility with antigen solutions of varying composition and pH
- Production of sterile adjuvants
- Easy to Use: Just mix with antigen and inject
Product Information
| Product Name | Description |
|---|---|
|
Gerbu Adjuvant MM for monoclonal antibody production |
Especially developed for monoclonal antibody production. High yield of IgG and fused clones in mice. Contains GMDP, Cimetidin. 0.1 ml for complete immunization of one animal. Single dose ca. 40 μl / mouse. |
|
Gerbu Adjuvant P for polyclonal antibody production in lab animals |
Laboratory adjuvant, predosed. Biocompatible and easy to handle: Just mix with antigen solution. Strongly active, for research purposes. Effect superior to FCA but less side effects. Becomes semisolid in refrigerator for long-time storage. Replaces GERBU Adjuvant LQ, 10 and 100. |
|
Gerbu Adjuvant FAMA for antibody production and vaccination of sensitive animals |
Extremely biocompatible and effective. For very sensitive animals such as horses and camels, cationized particles of tocopherol and lecithin plus GMDP and immunostimulators. Very stable. |
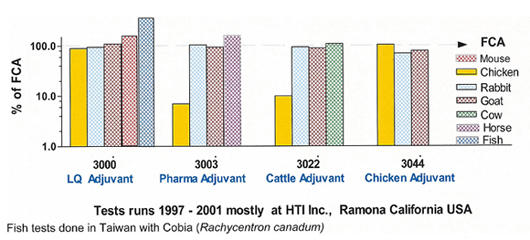
Comparison Gerbu Adjuvant Types
Instruction
- Gerbu Adjuvant MM (Adjuvant_MM_3001.pdf)
- Gerbu Adjuvant P (Adjuvant_P_3111.pdf)
- Gerbu Adjuvant FAMA (Adjuvant_FAMA_3030.pdf)
Reference
- GRUBHOFER, N. (2008). Vaccine Adjuvants revisited. The Open Veterinary Science Journal, 2, 63-67<
- GRUBHOFER, N. (1995). An adjuvant formulation based on GMDP and DDA synergist. Immunology Letters 44: 19-24
- PARDON, E., LAEREMANS, T., TRIEST, S., RASMUSSEN, S. G. F., WOHLKÖNIG, A., RUF, A., MUYLDERMANS, S., HOL, W.G.J., KOBILKA, B.K., & STEYAERT, J. (2014). A general protocol for the generation of Nanobodies for structural biology. Nature Protocols 9, 674-693.
- KATEREGGA, J., LUBEGA, G.W., LINDBLAD, E.B., AUTHIÉ, E., COETZER, T.H.T. & BOULANGÉ, A.F.V. (2012). Effect of adjuvants on the humoral immune response to congopain in mice and cattle. BMC Veterinary Research, 8:63.
- MARGOS, M.1, GOTTSTEIN, B. (2010). Gerbu adjuvant modulates the immune response and thus the course of infection in C56BL/6 mice immunised with Echinococcus multilocularis rec14-3-3 protein. Parasitology Research. Aug;107 (3) :623-9.
- FERBER, P.C., OSSENE, P., HOMBERGER, F.R., & FISCHER, R.W. (1999). The generation of monoclonal antibodies in mice: influence of adjuvants on the immune response, fusion efficiency and distress. Laboratory Animals, 33.
- Bundesamt für Veterinärwesen (2017). Fachgerechte und tierschutzkonforme Antikörperproduktion in Kaninchen, Hühnern und Labornagetieren. In: Fachinformation 3.04
- IVANOV, V.T., ANDRONOVA, T.M., BEZRUKOV, M.V., RAR, V.A., MAKAROV, E.A., KOZMIN, S.A., ASTAPOVA, M. V., BARKOVA, T.I., & NESMEYANOV, V.A. (1987). Structure, design, and synthesis of immunoactive peptides. Pure & Applied Chemistry, Vol. 59, No. 3, pp. 317--324.
- JOHANNSEN L., ROSENTHAL, R.S., MARTIN, S.A., CADY, A.B., OBAL, Jr. F., GUINAND M., KRUEGER, J.M. (1989). Somnogenic activity of O-acetylated and dimeric muramyl dipeptides. Infection and Immunity, 57(9):2726-2732.
Ordering Information
| Product Name | Storage | Product No. | PKG Size |
|---|---|---|---|
| Gerbu Adjuvant MM | Refrigerator | 3001.0106 | 6 x 0.1 ml |
| 3001.1000 | 1 ml | ||
| 3001.5000 | 5 ml | ||
| 3001.6001 | 6 x 1 ml | ||
| 3001.6005 | 6 x 5 ml | ||
| Gerbu Adjuvant P | Refrigerator | 3111.0001 | 1 ml |
| 3111.0005 | 5 ml | ||
| 3111.6001 | 6 x 1 ml | ||
| 3111.0025 | 25 ml | ||
| 3111.6005 | 6 x 5 ml | ||
| 3111.0100 | 100 ml | ||
| Gerbu Adjuvant FAMA | Refrigerator | 3030.0025 | 25 ml |
| 3030.0100 | 100 ml |