TUBE (Tandem Ubiquitin Binding Entity) (LifeSensors)

Ubiquitin Affinity Matrices (TUBEs)
The study of ubiquitylated proteins is complicated by the fact that these modified substrates are not stable either in cells or in vitro. In order to aid researchers in the purification and identification of ubiquitylated proteins LifeSensors licensed the TUBE (Tandem ubiquitin binding entity) technology from CICBioGUNE.
TUBEs have a superior affinity for poly-ubiquitin chains compared to traditional ubiquitin binding domains (up to 1000-fold increase in affinity). These TUBEs can be used to both stabilize poly-ubiquitin chains and purify ubiquitylated proteins making them a valuable addition to the ubiquitin researcher's toolbox.
Hjerpe, R. et al. Efficient protection and isolation of ubiquitylated proteins using tandem ubiquitin-binding entities. EMBO Rep. 10(11), 1250-1258 (2009).
Product Information
SUPERIOR, EASIER, PROTECTIVE
Up to 1000X higher affinity than a UBA
IP Ubiquitylated protein without overexpression or Proteasome Inhibitor
Protect ubiquitylated proteins from the degradation
TUBE 1 displays an approximately 10-fold higher affinity for K63 tetra-ubiquitin relative to K48 linked species, and may allow for enrichment of proteins that are polyubiquitylated through this linkage type.
TUBE 2 displays equivalent affinities for both K63 and K48 tetra-ubiquitin; therefore, this TUBE is a resonable starting point if the nature of the ubiquitin linkage is unknown.
TUBE 3 also has equivalent affinities for both K63 and K48 tetra-ubiquitin, however it's relative affinity for monoubiquitin is lower than that of either TUBE 1 or TUBE 2, allowing for greater sensitivity in detection of polyubiquitylated proteins.
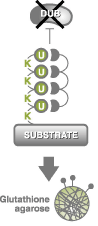
TUBEs are high affinity ubiquitin traps that bind to poly-ubiquitin chains.
They bind ubiquitin chains with low nanomolar affinity and can be used for inhibition of deubiquitylation and protein degradation; capture of total ubiquitylated proteins from cell cultures, tissues and organs; and many other uses.
Product lineup
横スクロール可能Scroll
| TUBE 1 | TUBE 2 | TUBE 3 | Application | ||
|---|---|---|---|---|---|
| Tag/Conjugate | |||||
| GST | UM101 | UM102 | -- | GST pulldown | |
| His6 | UM201 | UM202 | UM203 | His pulldown | |
| Biotin | UM301 | UM302 | -- | Far-Western | |
| Agarose | UM401 | UM402 | -- | Pulldown | |
Protocols
PULLDOWN OF POLYUBIQUITYLATED PROTEINS
- Pre-chill cell lysis buffer to 4°C.
- Add supplied TUBEs to 500 µl of lysis buffer to a final concentration of 100-200 µg/mL. (1.8-3 µM). Store on ice.
- Treat and wash cells appropriately, and add of 500 µL of lysis buffer to a 10cm tissue culture dish containing approximately 1.5x106 cells.
- Collect cells by scraping, and transfer lysate to a pre-chilled 1.5mL centrifuge tube.
- Incubate TUBEs containing lysate for 15 minutes on ice.
- Clarify cell lysate by centrifugation for 10 minutes at ~14,000xg (4°C).
- Collect supernatant.
- Capture TUBEs using glutathione affinity resin or IMAC resin.
PURIFICATION OF POLYUBIQUITYLATED PROTEINS
- Pre-chill cell lysis buffer to 4°C.
- Treat and wash cells appropriately, and add of 500 µL of lysis buffer to a 10cm tissue culture dish containing approximately 1.5x106 cells.
- Collect cells and lysate by scraping, and transfer to a pre-chilled 1.5mL micro centrifuge tube.
- Clarify lysate through high speed centrifugation (~14,000xg) for 10min.
- Add equilibrated Agarose-TUBEs (10-20 µl) to lysate.
- Incubate for 4 hours at 4°C.
- Clarify cell lysate by centrifugation for 10 minutes at ~14,000xg (4°C).
- Remove supernatant, wash Agarose-TUBEs with TBS-T.
- Treat resin with 0.2M glycine HCl, pH 2.5 for at least one 1 hour on a rocking platform (4°C).
- Collect resin by high speed centrifugation (13,000xg) for 5 minutes.
- Recover supernatant.
Applicaion
Purification of Poly-Ubiquitinated proteins using TUBE Technology
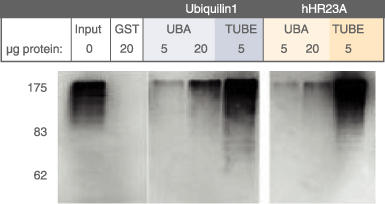
Ref; Hjerpe et al, EMBO Report, Oct. 2009
Far-western blotting using Biotin-TUBE1
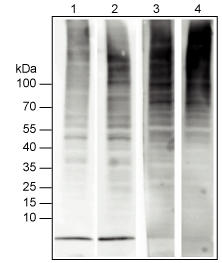
Approximately 40 µg of protein from Neura 2A cells was subjected to SDS-PAGE, prior to electrophoretic transfer.
- Strip1 : Probing with polyclonal a-ubiquitin without prior heating of membrane
- Strip2 : Probing with polyclonal a-ubiquitin with heating
- Strip3 : Probing with TUBE (0.2 µg/ml)without heating
- Strip4 : Probing with TUBE (1 µg/ml)without heating
Comparison of Agarose-TUBEs to similar technologies for the pulldown of total polyubiquitinated protein
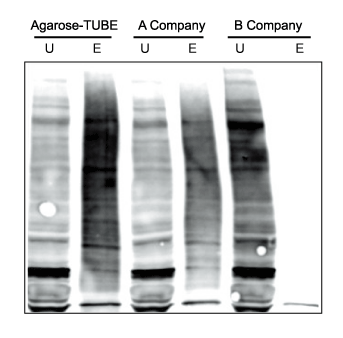
- U : Equivalent amounts of unbound
- E : Eluted
For each, total protein (~4mg) from HEK293T cells was incubated with 10 µl of resin. Equivalent amounts of unbound (U) and eluted (E) protein were analyzed by SDS-PAGE, with immunodetection by polyclonal anti-ubiquitin lgG.
Example
Pulldown of Total Polyubiquitinated Protein with Agarode-TUBE1
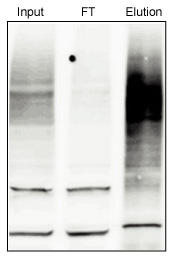
Approximately 5mg of total protein was isolated from HEK293T cells and subjected to pulldown with 10 µl of Agarose-TUBE 1. Input and Flow-through (1/10th of total) are shown for comparison. Protein was probed with anti-ubiquitin lgG.
Ordering Information
Pulldown of Total Polyubiquitinated Protein with Agarode-TUBE1
横スクロール可能Scroll
| Tag/Conjugate | Product Name | Storage | Product No. | Content |
|---|---|---|---|---|
| GST | GST-TUBE1 | -80°C | UM101 | 200 µg (5 mg/ml) |
| GST-TUBE2 | -80°C | UM102 | 200 µg (5 mg/ml) | |
| His6 | His6-TUBE1 | -80°C | UM201 | 200 µg (5 mg/ml) |
| His6-TUBE2 | -80°C | UM202 | 200 µg (5 mg/ml) | |
| His6-TUBE3 | -80°C | UM203 | 200 µg (5 mg/ml) | |
| Biotin | Biotin-TUBE1 | -80°C | UM301 | 200 µg (5 mg/ml) |
| Biotin-TUBE2 | -80°C | UM302 | 200 µg (5 mg/ml) | |
| Agarose | Agarose-TUBE1 | -20°C | UM401 | 1 ml (50%slurry) |
| Agarose-TUBE2 | -20°C | UM402 | 1 ml (50%slurry) |
(Storage) RT: Room temperature, A: Cool and dark, R: Refrigerator, F: Freeze