COSMOSIL SFC Columns

Supercritical Fluid Chromatography (SFC) has become more attractive because it offers some advantages over HPLC, such as high speed, unique selectivity and environmentally friendly separations. Many conventional normal-phase stationary phases, such as diol, amino and cyano, have been used for SFC applications. However, these phases present limitations for separations. COSMOSIL SFC Columns have been developed to enhance the capability of SFC separations.
Features:
- Three categories (I–III) of stationary phase for different types of compounds
- Different selectivity from HPLC
Product Information
Category I: Columns for mid- to high-polarity compounds
For these compounds, a high-polarity stationary phase is suitable. More polar compounds are retained longer.
| Product | Stationary phase | Features |
| COSMOSIL PY | Pyridinyl | Similar selectivity to 2-ethylpyridine; strong retention in general. |
| COSMOSIL HP | 3-Hydroxyphenyl | Different selectivity from PY; strong retention for basic compounds. |
| COSMOSIL Diol | Diol | Less effect from ionic interaction. |
Category II: Columns for low-polarity compounds
For these compounds, a low-polarity stationary phase is suitable.
| Product | Stationary phase | Features |
| COSMOSIL Cholester | Cholesteryl | Longer retention and better separation than C18. |
Category III: Columns for SFC-specific separations
In supercritical fluid chromatography (SFC), secondary interactions such as π-π and dispersion force* are stronger compared to high-performance liquid chromatography (HPLC). As a result, these columns are capable of unique separations in SFC.
| Product | Stationary phase | Features |
| COSMOSIL πMAX | Pyrenylethyl | Stronger π-π interactions than phenyl columns. |
| COSMOSIL PBr | Pentabromobenzyl | Unique separations using dispersion force.* |
* Dispersion force:
London dispersion force is a weak intermolecular force that results from dipoles temporarily induced from random unsymmetrical electron positions in two adjacent atoms, also known as "instantaneous dipole-induced dipole force". It is present in all molecules, regardless of whether they are polar or non-polar. Compounds with high polarizability have stronger dispersion force.
Selection by Polarity
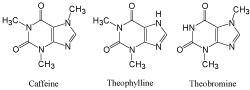
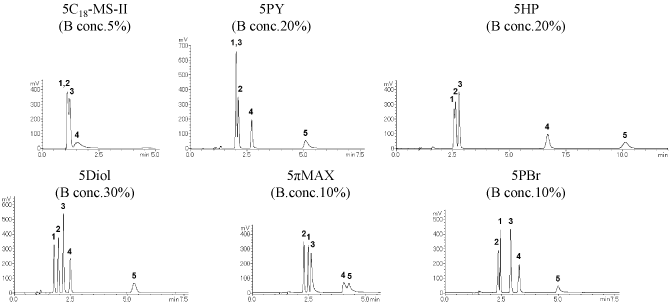
Derivatives of xanthine (high-polarity compounds) XLogP3: -0.8 – 0.0
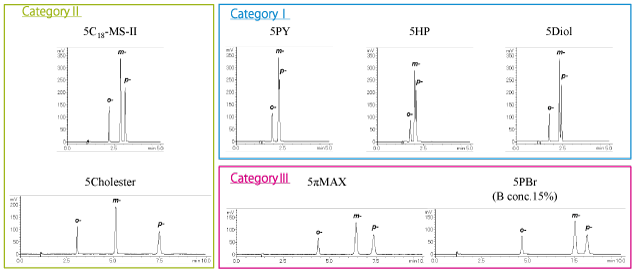
Category I columns (COSMOSIL 5PY, 5HP, 5Diol) and COSMOSIL 5πMAX separated the sample well. The elution order with COSMOSIL 5πMAX was different than the category I columns.
| Conditions | |||
|---|---|---|---|
| Column | COSMOSIL ** | BPR | 10 MPa |
| Column size | 4.6 mmI.D. × 250 mm | Temp. | 40°C |
| Detection | UV 273 nm | ||
| Mobile phase | A:CO2 B:Methanol B conc. 10%(πMAX only:20%) |
Sample |
|
| Flow rate | 3.0 mL/min | Inj. vol. | 5.0 µL |
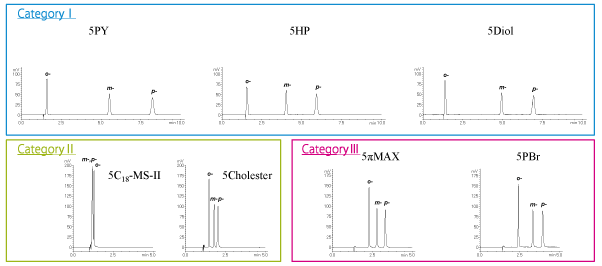
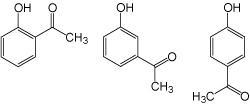
Positional isomers (mid-polarity compounds) XLogP3: 1.4 – 1.9
Category I columns (COSMOSIL 5PY, 5HP, 5Diol) yielded the best separation.
| Conditions | |||
|---|---|---|---|
| Column | COSMOSIL ** | BPR | 10 MPa |
| Column size | 4.6 mmI.D. × 250 mm | Temp. | 40°C |
| Detection | UV 254 nm | ||
| Mobile phase | A:CO2 B:Methanol B conc. 5% |
Sample |
o-Hydroxyacetophenone (0.1 mg/mL) m-Hydroxyacetophenone (0.1 mg/mL) p-Hydroxyacetophenone (0.1 mg/mL)
|
| Flow rate | 3.0 mL/min | Inj. vol. | 5.0 µL |
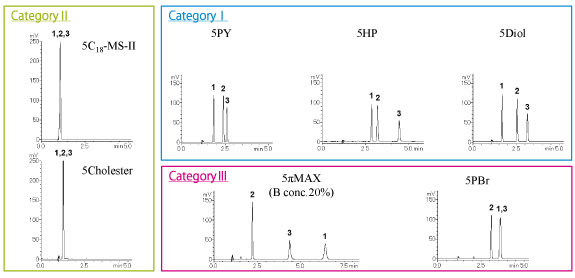
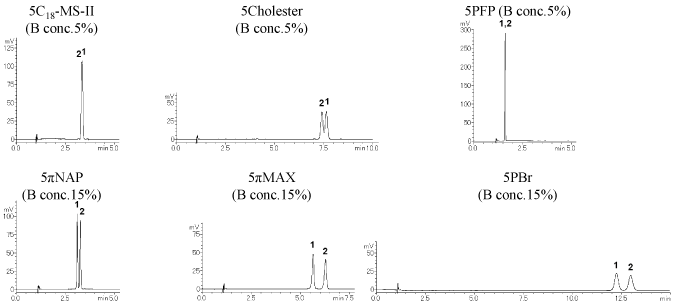
Positional isomers (low-polarity compounds) XLogP3: 5.8 – 6.0
Category III (COSMOSIL 5πMAX, 5PBr) and category II (5Cholester) columns separated most effectively.
| Conditions | |||
|---|---|---|---|
| Column | COSMOSIL ** | BPR | 10 MPa |
| Column size | 4.6 mmI.D. × 250 mm | Temp. | 40°C |
| Detection | UV 254 nm | ||
| Mobile phase | A:CO2 B:Methanol B conc. 5%(PBr only: 15%) |
Sample |
o-Terphenyl(0.1 mg/mL) m-Terphenyl(0.1 mg/mL) p-Terphenyl(0.1 mg/mL)  |
| Flow rate | 3.0 mL/min | Inj. vol. | 5.0 µL |
Applications
Vitamin D
COSMOSIL 5πMAX and 5PBr were able to separate the vitamins. The PFP (pentafluorophenyl) column, which also uses a halogenated stationary phase, could not separate them. The dispersion force used by PBr interacts more strongly with larger molecules. PFP, which is a smaller molecule, does not exhibit this selectivity.
| Conditions | |||
|---|---|---|---|
| Column | COSMOSIL ** | BPR | 10 MPa |
| Column size | 4.6 mmI.D. × 250 mm | Temp. | 40°C |
| Detection | UV 265 nm | ||
| Mobile phase | A:CO2 B:Methanol B conc. **% |
Sample |
|
| Flow rate | 3.0 mL/min | Inj. vol. | 3.0 µL |
Nucleobases
COSMOSIL 5Diol separated 5 nucleic acid bases.
| Conditions | |||
|---|---|---|---|
| Column | COSMOSIL ** | BPR | 10 MPa |
| Column size | 4.6 mmI.D. × 250 mm | Temp. | 40°C |
| Detection | UV 260 nm | ||
| Mobile phase | A:CO2 B:100 mmol/L Ammonium Acetate -Methanol B conc. **% |
Sample |
|
| Flow rate | 3.0 mL/min | Inj. vol. | 5.0 µL |
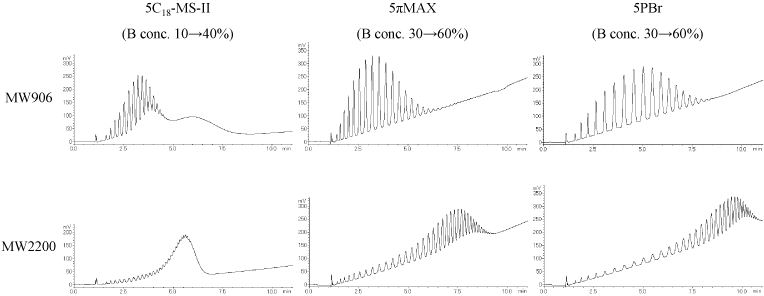
Polystyrene
These polystyrene samples with different degrees of polymerization are likely separated by number of monomer units. COSMOSIL 5πMAX and 5PBr were able to separate the high-MW polystyrene.
| Conditions | |||
|---|---|---|---|
| Column | COSMOSIL ** | BPR | 10 MPa |
| Column size | 4.6 mmI.D. × 250 mm | Temp. | 40°C |
| Detection | UV 220 nm | ||
| Mobile phase | A:CO2 B:Tetrahydrofuran B conc. *→*% 10 min Linear gradient |
Sample |
Polystyrene, MW906 (5.0 mg/mL) Polystyrene, MW2,200 (5.0 mg/mL)
|
| Flow rate | 3.0 mL/min | Inj. vol. | 5.0 µL |
Surfactants

| Conditions | |||
|---|---|---|---|
| Column | COSMOSIL 5PBr | BPR | 10 MPa |
| Column size | 4.6 mmI.D. × 250 mm | Temp. | 40°C |
| Detection | UV 220 nm | ||
| Mobile phase | A:CO2 B:Methanol B conc.2→100% 10min Linear gradient |
Sample |
Triton X-100 (5.0 mg/mL)
|
| Flow rate | 2.0 mL/min | Inj. vol. | 5.0 µL |
Non-steroidal anti-inflammatory drugs
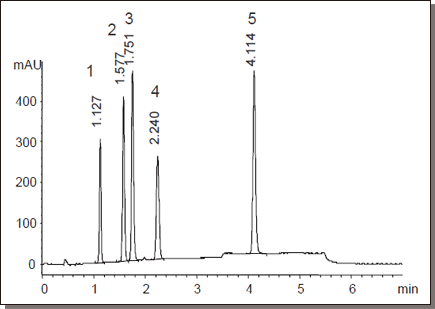
| Conditions | ||||
|---|---|---|---|---|
| Column | COSMOSIL HP | Sample |
|
|
| Column size | 4.6 mm I.D. x 150 mm | |||
| Mobile phase | Carbon Dioxide : Methanol | |||
| Gradient | 95:5 to 65:35 over 6 minutes | |||
| Flow rate | 5.0 ml/min | |||
| Temperature | 30°C | |||
| Detection | UV 230 nm | |||
Beta Blockers
Peak elution order reversed under identical conditions.
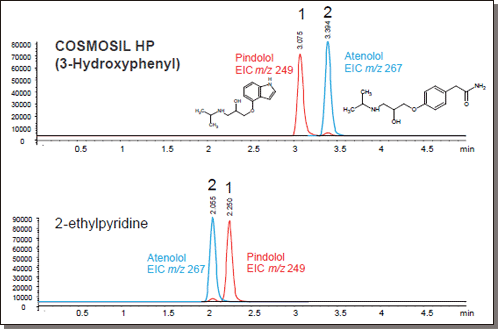
| Conditions | ||||
|---|---|---|---|---|
| Column | COSMOSIL HP and a 2-ethylpyridine column | Sample |
|
|
| Column size | 4.6 mm I.D. x 150 mm | |||
| Mobile phase | Carbon Dioxide : Methanol w/20 mM ammonium formate |
|||
| Gradient | 95:5 to 50:50 over 4 minutes | |||
| Flow rate | 5.0 ml/min | |||
| Temperature | 30°C | |||
| Detection | APCI(+) | |||
Hydrophilic organics
COSMOSIL PY (Pyridinyl)
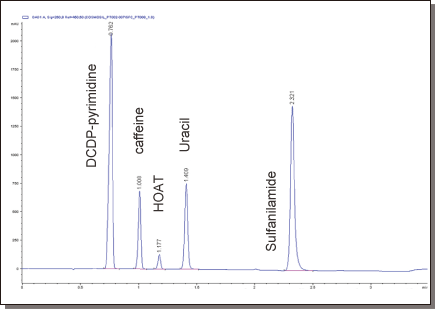
| Conditions | ||||
|---|---|---|---|---|
| Column size | 4.6 mm I.D. x 150 mm | |||
| Flow rate | 5.6 mL/min | |||
| Pressure | 140 bar | |||
| Temperature | Ambient | |||
| Mobile phase | Methanol gradient 5-50% @18%/minute; hold @ 50% 0.1 min; return to 5% @99%/min |
|||
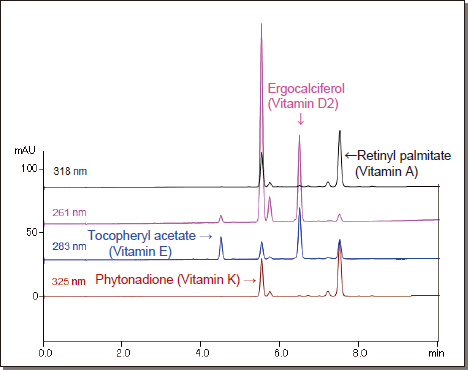
Fat-Soluble Vitamin Analysis
COSMOSIL Cholester
When used with SFC, COSMOSIL Cholester can separate fat-soluble vitamins and their impurities.
| Conditions | ||||
|---|---|---|---|---|
| Column size | 4.6 mm I.D. x 250 mm | |||
| Mobile phase | IPA in CO2 IPA conc. 0→10%(0–10min),10%(10–12min) |
|||
| Flow rate | 3.0 ml/min | |||
| BPR | 15 MPa | |||
| Temperature | 40°C | |||
| Detection | UV-VIS | |||
| Sample conc | 2.0 mg/ml | |||
| Inj. Vol | 1.0 µl | |||
| Sample | 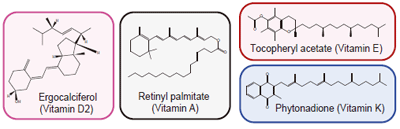 |
|||
COSMOSIL Cholester exhibits strong retention for fat-soluble vitamins and
is suitable for on-line SFE-SFC using Shimadzu’s Nexera UC. The on-line
extraction from food also produced triglyceride impurities, which were
successfully separated from the
vitamins.
Data courtesy of Shimadzu Corporation
Column Comparison Data
Amitriptyline
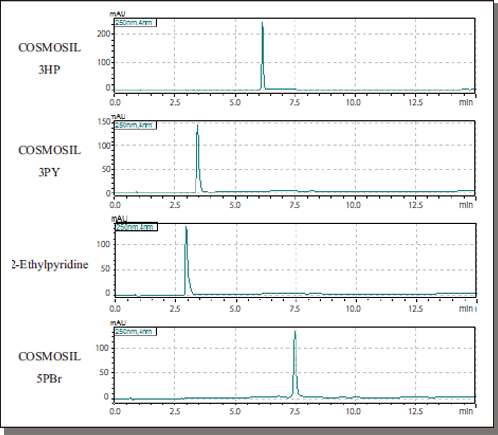
| Conditions | ||||
|---|---|---|---|---|
| Column size | 2.1 mm I.D. × 150 mm | Temperature | 40°C | |
| Mobile phase | A: CO2 B: 0.1% CH3COONH4 -Methanol B conc. 0→60% (0–14 min), 60% (14–17 min) |
Detection | UV 250 nm | |
| Inj.Vol.: | 2.0 µl | |||
| Sample | Amitriptyline (1 mmol/l)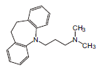 |
|||
| Flow rate | 0.8 ml/min | |||
| BPR | 10 MPa | |||
Data courtesy of Kyushu University Medical Institute of Bioregulation Research Center for Transomics Medicine Division of Metabolomics
Reserpine
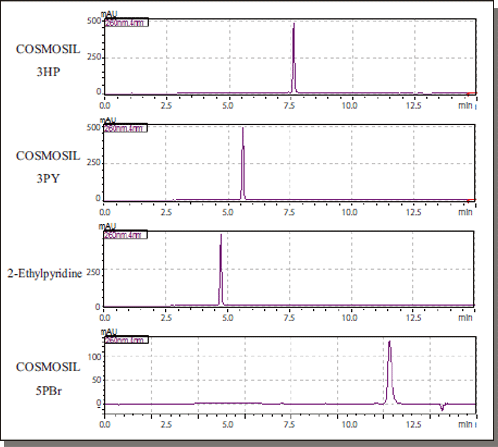
| Conditions | ||||
|---|---|---|---|---|
| Column size | 2.1 mm I.D. × 150 mm | BPR | 10 MPa | |
| Mobile phase | A: CO2 B: 0.1% CH3COONH4 -Methanol B conc. 0→60% (0–14 min), 60% (14–17 min) |
Temperature | 40°C | |
| Detection | UV 260 nm | |||
| Inj. Vol. | 2.0 µl | |||
| Flow rate | 0.8 ml/min | |||
| Sample | Reserpine (1mmol/l)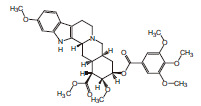 |
|||
Data courtesy of Kyushu University Medical Institute of Bioregulation Research Center for Transomics Medicine Division of Metabolomics
3-Aminobenzoic Acid
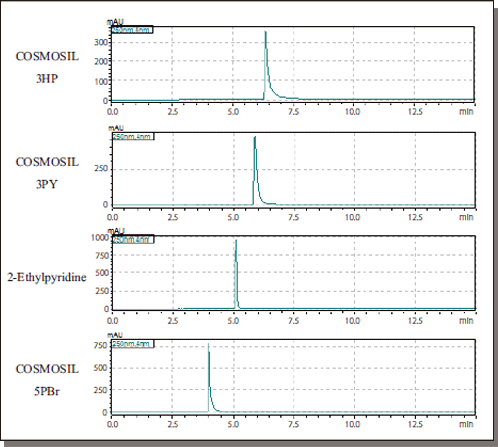
| Conditions | ||||
|---|---|---|---|---|
| Column size | 2.1 mm I.D. × 150 mm | BPR | 10 MPa | |
| Mobile phase | A: CO2 B: 0.1% CH3COONH4 -Methanol B conc. 0→60% (0–14 min), 60% (14–17 min) |
Temperature | 40°C | |
| Detection | UV 250 nm | |||
| Inj.Vol. | 2.0 µl | |||
| Flow rate | 0.8 ml/min | |||
| Sample | 3-Aminobenzoic Acid (10mmol/l) 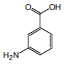 |
|||
Data courtesy of Kyushu University Medical Institute of Bioregulation Research Center for Transomics Medicine Division of Metabolomics
Benzoic Acid
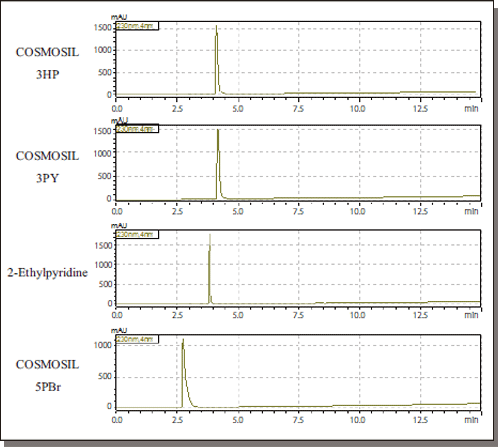
| Conditions | ||||
|---|---|---|---|---|
| Column size | 2.1 mm I.D. × 150 mm | BPR | 10 MPa | |
| Mobile phase | A: CO2 B: 0.1% CH3COONH4 -Methanol B conc. 0→60% (0–14 min), 60% (14–17 min) |
Temperature | 40°C | |
| Detection | UV 230nm | |||
| Inj. Vol. | 2.0 µl | |||
| Flow rate | 0.8 ml/min | |||
| Sample | Benzoic Acid (10 mmol/l) |
|||
Data courtesy of Kyushu University Medical Institute of Bioregulation Research Center for Transomics Medicine Division of Metabolomics
Packing Material Properties
| Packing material | PY | HP | Diol | Cholester | πMAX | PBr |
|---|---|---|---|---|---|---|
| Silica gel | High-purity porous spherical silica | |||||
| Average particle size (µm) | 3, 5 | 5 | 5 | 5 | 5 | |
| Average pore size (Å) | 120 | |||||
| Stationary phase | 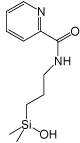 |
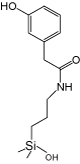 |
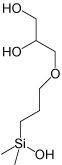 |
 |
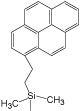 |
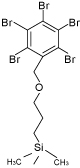 |
| Pyridinyl | 3-Hydroxyphenyl | Diol | Cholesteryl | Pyrenylethyl | Pentabromobenzyl | |
| Endcapping treatment | Endcapped | |||||